Cancer is a leading cause of death worldwide with more than 10 million deaths per year [1]. Despite significant advances in cancer research, current modalities such as chemotherapy, radiation, and surgery have limitations due to their nonspecific targeting and significant side effects. A relatively new approach to combat cancer, called immunotherapy, aims to stimulate the immune system to recognize and destroy only cancer cells, leaving healthy cells intact. One of the most promising approaches to immunotherapy involves the use of T cells that have been genetically engineered to express chimeric antigen receptors (CARs) that recognize tumor-associated antigens.
The CRISPR‑Cas9 system is a precise and efficient gene‑editing tool that is being utilized to develop CAR‑T cells with enhanced antitumor activity. CRISPR editing enables the specific customization of T cells to enhance their functionality and improve their ability to recognize and destroy cancer cells.
What are CAR-T cells?
Normal T cells mediate key parts of the adaptive cell-mediated immune response. Before they mature and release from the thymus, T cell receptors must go through a checkpoint screening. During this process, the thymocytes which recognize “self” antigens are removed by apoptosis while those that recognize “non-self” antigens mature and move to the spleen or lymph nodes—where they are exposed to foreign antigens via the major histocompatibility complex (MHC) of antigen-presenting cells. In addition, activated T cells release cytokines such as IFN‑gamma, TNF‑alpha, and TNF‑beta.
In contrast to normal T cells, chimeric antigen receptor (CAR) T cells are engineered via either viral delivery or non-viral methods to express a recombinant gene for CAR, consisting of three domains: (1) extracellular domain with the antigen recognition region; (2) transmembrane domain; and (3) the intracellular domain that has three immunoreceptor tyrosine-based activation motifs (ITAMs), costimulatory molecules (CM1) such as CD28, and an interleukin-12 (IL-12) domain that stimulates the innate immune system (Figure 1).
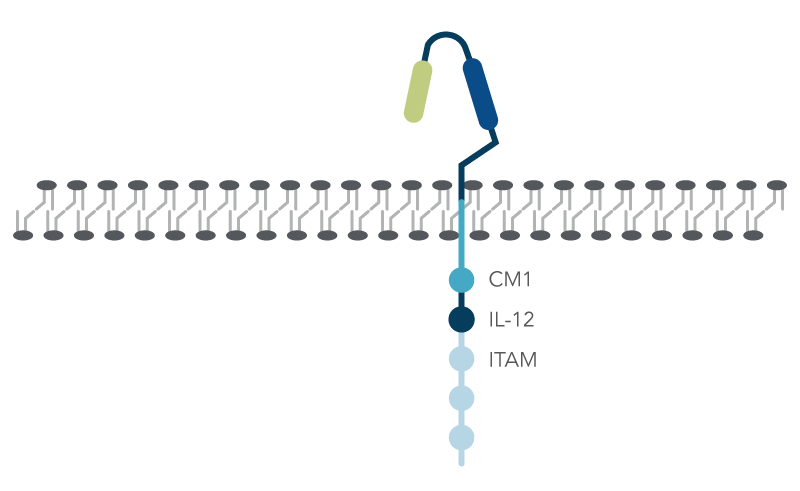
How does CRISPR-Cas9 gene editing create the desired CAR-T cells?
The CRISPR‑Cas9 system is an efficient gene-editing technique that can be used for “knocking in” or “knocking out” genes. Researchers can use CRISPR to insert CAR genes in a precise location of the T‑cell genome. T‑cell receptor alpha chain (TRAC) is a region of the genome consisting of the endogenous T‑cell receptor gene. The CRISPR‑Cas9 system can efficiently edit the existing T‑cell receptor gene through the insertion of the tumor-targeting CAR gene. Experiments have demonstrated that the insertion of CAR genes in the TRAC region using the CRISPR-Cas9 system shows high CAR expression and improved anti-tumor activity [2].
Researchers would like to find ways for the CAR‑T cells to be standardized, a so called “off-the-shelf” allogenic source. Developing new technology is necessary to eliminate potential immune responses which can contribute to the risk of rejection. Universal allogeneic T cells have been created using CRISPR‑Cas9 gene editing to target programmed cell death 1 (PD-1), T cell receptor alpha constant (TRAC), and beta‑2‑microglobulin (β2M). Targeting PD‑1 and TRAC has been shown to improve T cell effector activities which abrogates tumor growth and improves CAR‑T cell efficacy [3,4]. Moreover, researchers determined that targeting β2M, a key subunit of the human leukocyte antigen class‑1 (HLA‑1) protein, allows for a higher persistence of CAR‑T cells in vivo [3].
What are the advantages of CRISPR‑Cas9 and CAR‑T compared to other immunotherapies such as immune checkpoint inhibitors?
FDA‑approved cancer immunotherapies include adoptive T cell therapy (ACT) and immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte antigen 4 (CTLA4), programmed cell death ligand (PD‑L1), and PD-1. The activation of these receptors can initiate an immune response leading to adverse effects [5].
The CRISPR‑Cas9 gene editing technique has been demonstrated to generate CAR‑T cells with precise antigen recognition, minimal toxicity, anti‑tumor effects, stimulate cytokine production, and inhibit checkpoint molecules [6,7].
What are the remaining challenges of using CRISPR‑Cas9 technique for generating CAR‑T cells?
Although the development of CAR-T cells using universal allogeneic T cells is promising, there are still several challenges that need to be addressed. This includes developing a simplified protocol for delivering sgRNAs and Cas9 while maintaining cell survival after gene manipulation. Also, the potential risks of using retroviruses, lentiviruses, adenovirus‑associated viruses (AAV) as viral vectors for delivering CRISPR‑Cas9 components to cells. Other challenges of CRISPR‑Cas9‑mediated editing of CAR‑T cells are off‑target effects and toxicity. Unintended editing could occur at locations other than the target sites in the genome. These off‑target effects can lead to unintended changes in gene expression, translocations, and gross chromosomal aberrations which potentially causes toxicity and other adverse effects [8]. Therefore, it is essential to carefully design and validate CRISPR‑Cas9‑mediated edits to minimize the risk of off-target effects in CAR‑T cells.
Conclusion
CRISPR-Cas9 gene editing is a more effective approach for manufacturing CAR‑T cells using universal “off-the-shelf” allogeneic T cells. Performing CRISPR‑Cas9 for generating CAR‑T cells is promising; however, challenges do exist when considering this approach.
Download our CRISPR Therapeutics eBook
CRISPR Therapeutics (PDF) is designed to explore the vast possibilities of Genomic Medicine. It will help translational research and cell and gene therapy developers discover what we have to offer with our cGMP/q7 capabilities for developing CRISPR-Cas9 gene therapies.
Download your copy of CRISPR Therapeutics eBook.


